|
Perry Cohen, Ph.D. presents Sham Neurosurgical Procedures in Clinical Trials: Patient Activists’ Perspectives at the NIH conference
Sham Neurosurgical Procedures in Clinical Trials for Neurodegenerative Diseases: Scientific and Ethical Considerations
held on June 30 and July 1, 2010
Page 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 9 - 10 - 11 - 12 - 13
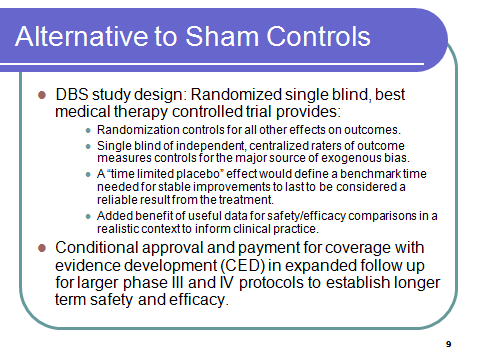
Alternative Design: Randomized, single blind, best medical therapy controlled
Drawing on controlled studies of DBS, this design avoids the ethical and methodological issues of the sham control group by randomization to surgical treatment versus best medical therapy. It provides a more realistic comparison for safety data (better information for actual choices) than the artificial comparison with the placebo control group which has been established entirely to neutralize the ‘bias’ from the very strong placebo response for brain surgery. The DBS studies quantify the additional risk from DBS versus usual care. Sham surgery does not provide this information.
SINGLE BLIND – of independent raters is used to control the major source of bias, which is the rating of subjective measures of the primary outcome UPDRS.
What we don’t know is whether ALL the effects of the experimental treatment are from placebo effects. Waiting for these placebo effects to wear off for at least a year seems prudent. If the effect lasts two years or more, it would not matter to most patients where it came from, it still could be worth the risk of the surgery. Clearly more research is needed.
A time limited placebo effect may need to define a benchmark for the amount of time necessary for stable improvements to be considered reliable. It would be established based on empirical research to determine the distribution of the length and quality of placebo response among PD patients, qualified by age, co-morbidities, severity of illness, and other relevant factors.
Alternative Regulatory Pathways
This “patient-centered” study design offers an alternative pathway to full FDA approval which is in keeping with the total life cycle regulatory responsibilities of the agency. It allows shorter, but easier steps that keep the process moving with conditional approvals and expanded protocols at each step. These timeframes would be long enough for placebo effects of treatment groups wear off and to expand the studies to wider populations and thus offer more safety data than would be currently forth-coming
It can also offer earlier revenue streams by coverage with evidence development (CED) to ease the up front capital requirements that weigh heavily in business decisions to make otherwise safe and effective treatments available to patients. This concept can be tested under current FDA authority, but PDUFA 5, now starting to be considered is a legislative vehicle to reinforce the implementation of such a test case.
Go Back Go Forward
Page 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 9 - 10 - 11 - 12 - 13
|
